Abstract
CD47 expressed on cells functions as a "don't eat me" signal by binding SIRPa on macrophages and blocking phagocytosis. Cancer cells including AML evade phagocytosis by overexpressing CD47, and high CD47 expression in AML has been associated with inferior outcomes. Here we studied the pre-clinical activity of the 5F9 (magrolimab), an antibody that blocks the CD47, combined with BCL-2 inhibitor venetoclax with azacitidine (VEN/AZA) in AML both in vitro and in vivo.
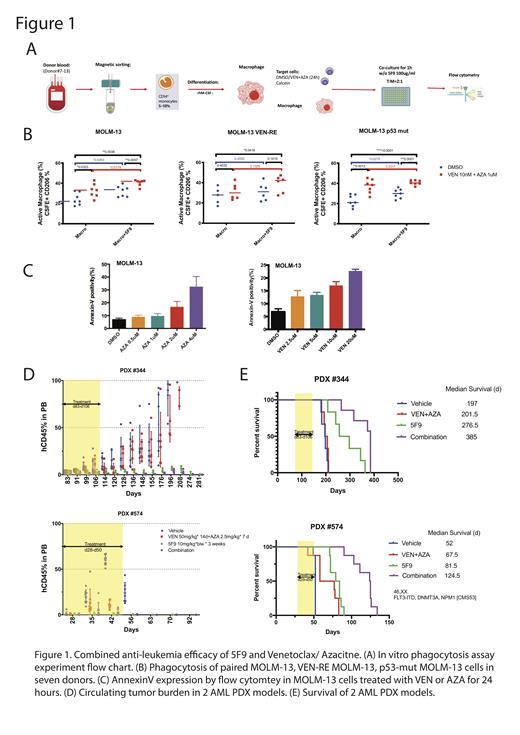
We first tested the combined efficacy of VEN/AZA and 5F9 in vitro in phagocytosis assay, utilizing monocytes isolated from seven healthy donors by CD16 positive selection and inducing macrophage differentiation with recombinant human M-CSF (rhM-CSF, 500ng/ml) for 1 week. Three paired isogenic AML cell lines MOLM-13, VEN-resistant (VEN-RE) MOLM-13, and p53-mutant MOLM-13 harboring R248W mutation, were pre-treated with DMSO or combination of 10nM VEN and 1uM AZA for 24hrs. Leukemia cells were labeled with Calcein and co-cultured with macrophages for 1h at a ratio of 2:1 (T:M), followed by flow cytometry read-out, quantifying Calcein+ macrophages that engulfed leukemia cells. The active phagocytosis was calculated as the percentage of Calcein+CD206+ cells from total CD206+ cells (Figure 1A, experimental schema). The 5F9/VEN-AZA combination significantly increased phagocytosis not only in parental but also in VEN-RE and p53-mutant MOLM-13 cells when compared with 5F9 or VEN/AZA alone treatments (Figure 1B). The treatment of VEN or AZA induced AnnexinV in MOLM-13 cells which may function as a pro-phagocytic signal and contribute to the enhanced phagocytosis (Figure 1C). Further proteomics studies to identify additional "eat me" signals following VEN/AZA treatment are ongoing and will be presented.
Next, we examined the therapeutic efficacy of 5F9 alone or combined with VEN/AZA in the AML patient-derived xenografts (PDX) models. NSG mice were engrafted with VEN-RE AML PDX #344 (no canonical AML mutations detected by targeted sequencing) and #574 (FLT3-ITD, DNMT3A, NPM1-mutated). After confirmed bone marrow (BM) engraftment by flow cytometry, mice were randomized to receive vehicle, 5F9 (10mg/kg/d, ip. twice a week, 3 weeks), VEN (50mg/kg/g, po. qd, 14 days) / AZA (2.5mg/kg/d, ip, qd, 7 days), or their combination. Circulating leukemia burden was monitored by hCD45 flow cytometry in peripheral blood (PB) (Figure 1D), and mice survival was followed. 5F9 reduced leukemia burden, delayed leukemia progression and significantly extended mice' survival in both models, with the median survival of 276.5 days of 5F9 group compared to 197 days of vehicle in #344, and 81.5 vs 52 days in #574 (Figure 1E ). On the contrary, VEN/AZA therapy had no or minimal impact on tumor burden or survival. Notably, the combination of 5F9 and VEN/AZA resulted in elimination of circulating blasts and significant extension of survival in both models, median of 385 days in #344 and 124.5 days in #574.
In summary, the VEN/AZA combined with 5F9 increased phagocytosis in isogenic MOLM-13 cells in vitro regardless of VEN resistance or p53 status and prolonged the survival in vivo in VEN/AZA refractory AML PDX models. The mechanisms of increased phagocytosis by combination treatment and the induction of "eat-me signals" by VEN/AZA is currently under investigation and will be presented. This triplet combination is currently being tested in a clinical trial (NCT04435691, Daver et al., ASH 2021) with high preliminary efficacy.
Chao: Gilead Sciences, Inc.: Current Employment; TigaTx: Membership on an entity's Board of Directors or advisory committees; Stanford University: Patents & Royalties; Hepatx Inc: Consultancy, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Iconovir Bio: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Leukemia and Lymphoma Society: Membership on an entity's Board of Directors or advisory committees; Stanford University Medical School: Membership on an entity's Board of Directors or advisory committees; Foresite capital: Consultancy; Bioverge: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Chimera Bioengineering: Current equity holder in publicly-traded company. Maute: Gilead Sciences: Ended employment in the past 24 months. Andreeff: Syndax: Consultancy; Karyopharm: Research Funding; ONO Pharmaceuticals: Research Funding; Senti-Bio: Consultancy; AstraZeneca: Research Funding; Daiichi-Sankyo: Consultancy, Research Funding; Breast Cancer Research Foundation: Research Funding; Medicxi: Consultancy; Aptose: Consultancy; Reata, Aptose, Eutropics, SentiBio; Chimerix, Oncolyze: Current holder of individual stocks in a privately-held company; Oxford Biomedica UK: Research Funding; Novartis, Cancer UK; Leukemia & Lymphoma Society (LLS), German Research Council; NCI-RDCRN (Rare Disease Clin Network), CLL Foundation; Novartis: Membership on an entity's Board of Directors or advisory committees; Glycomimetics: Consultancy; Amgen: Research Funding. Daver: Hanmi: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Gilead Sciences, Inc.: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Novimmune: Research Funding; Trillium: Consultancy, Research Funding; Glycomimetics: Research Funding; ImmunoGen: Consultancy, Research Funding; Sevier: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; FATE Therapeutics: Research Funding; Trovagene: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Consultancy; Jazz Pharmaceuticals: Consultancy, Other: Data Monitoring Committee member; Dava Oncology (Arog): Consultancy; Celgene: Consultancy; Syndax: Consultancy; Shattuck Labs: Consultancy; Agios: Consultancy; Kite Pharmaceuticals: Consultancy; SOBI: Consultancy; STAR Therapeutics: Consultancy; Karyopharm: Research Funding; Newave: Research Funding. Konopleva: Rafael Pharmaceuticals: Other: grant support, Research Funding; Novartis: Other: research funding pending, Patents & Royalties: intellectual property rights; Ascentage: Other: grant support, Research Funding; Reata Pharmaceuticals: Current holder of stock options in a privately-held company, Patents & Royalties: intellectual property rights; Eli Lilly: Patents & Royalties: intellectual property rights, Research Funding; F. Hoffmann-La Roche: Consultancy, Honoraria, Other: grant support; KisoJi: Research Funding; Stemline Therapeutics: Research Funding; Ablynx: Other: grant support, Research Funding; AstraZeneca: Other: grant support, Research Funding; Cellectis: Other: grant support; Sanofi: Other: grant support, Research Funding; Calithera: Other: grant support, Research Funding; Genentech: Consultancy, Honoraria, Other: grant support, Research Funding; Agios: Other: grant support, Research Funding; Forty Seven: Other: grant support, Research Funding; AbbVie: Consultancy, Honoraria, Other: Grant Support, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal